In a national poll conducted just days before the FDA announcement about the Pfizer vaccine, more than one-third of unvaccinated adults (35%) said the FDA’s full approval of one of the vaccines would address most or all of their concerns about the vaccines’ safety and effectiveness. Another 20% said it would address “some” of their concerns. The poll — conducted August 19-22 by data intelligence company Morning Consult on behalf of the de Beaumont Foundation — also identified messages that would make unvaccinated adults feel more confident in the vaccines following FDA approval. A quarter of the respondents said the following messages would make them somewhat more confident or much more confident:
- FDA’s full approval of the first COVID-19 vaccine is an important milestone that should reassure anyone who has concerns about getting vaccinated.
- All three COVID-19 vaccines have been proven to be safe and effective, based on extensive clinical trials and the fact that nearly 200 million Americans have received at least one shot without major complications.
- With the FDA granting full approval for a COVID-19 vaccine, Americans can be even more confident that the COVID-19 vaccines work and are safe.
- All three COVID-19 vaccines work. They reduce the risk of getting COVID-19 and greatly reduce your risk of being hospitalized or dying.
- For people who have said they will “wait and see,” the results are in — the COVID-19 vaccines work, and they’re safe.
When asked which of nine descriptions of FDA approval would give them the most confidence in the vaccines’ safety and effectiveness, unvaccinated adults favored a statement that focused on extensive data. These were the top messages:
- For full approval of a new drug, the FDA requires extensive data on safety and effectiveness, inspection of manufacturing facilities, and a comprehensive review of all clinical and “real-world” use.
- When a vaccine receives the FDA’s full approval, it’s no different than the shot people have been getting for months. It just means we have even more data proving that it works and is safe.
- FDA’s full approval means that a vaccine has cleared every level of review.
- Full approval represents the FDA’s highest level of confidence in a drug’s safety and effectiveness.
From a list of options, respondents were asked to choose what would make the biggest impact on their decision to get a COVID-19 vaccine. A full 39% of unvaccinated adults chose FDA approval as their first or second choice, followed by employer requirements, less testing, access to public events, and encouragement from their children.
Which of the following would make you most likely to be vaccinated against COVID-19?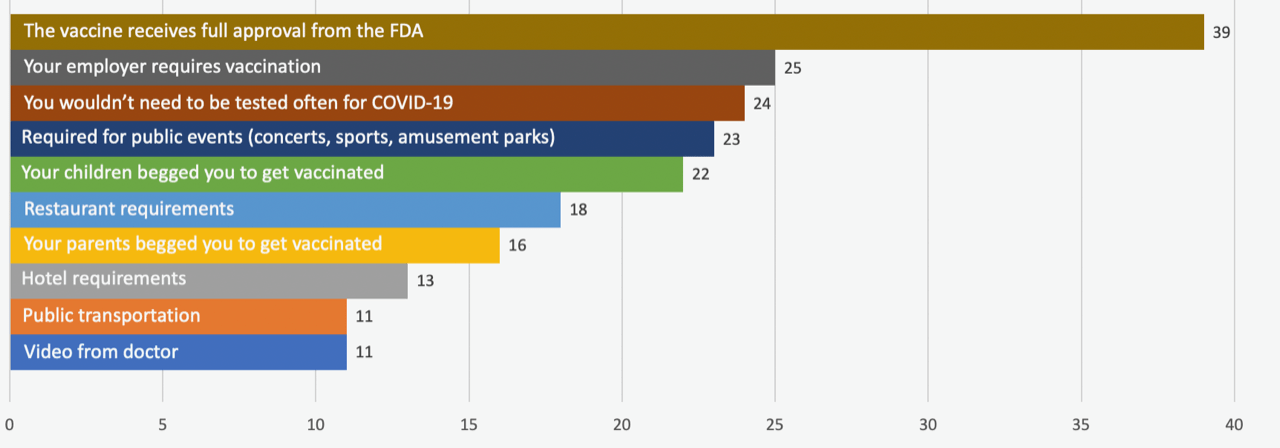
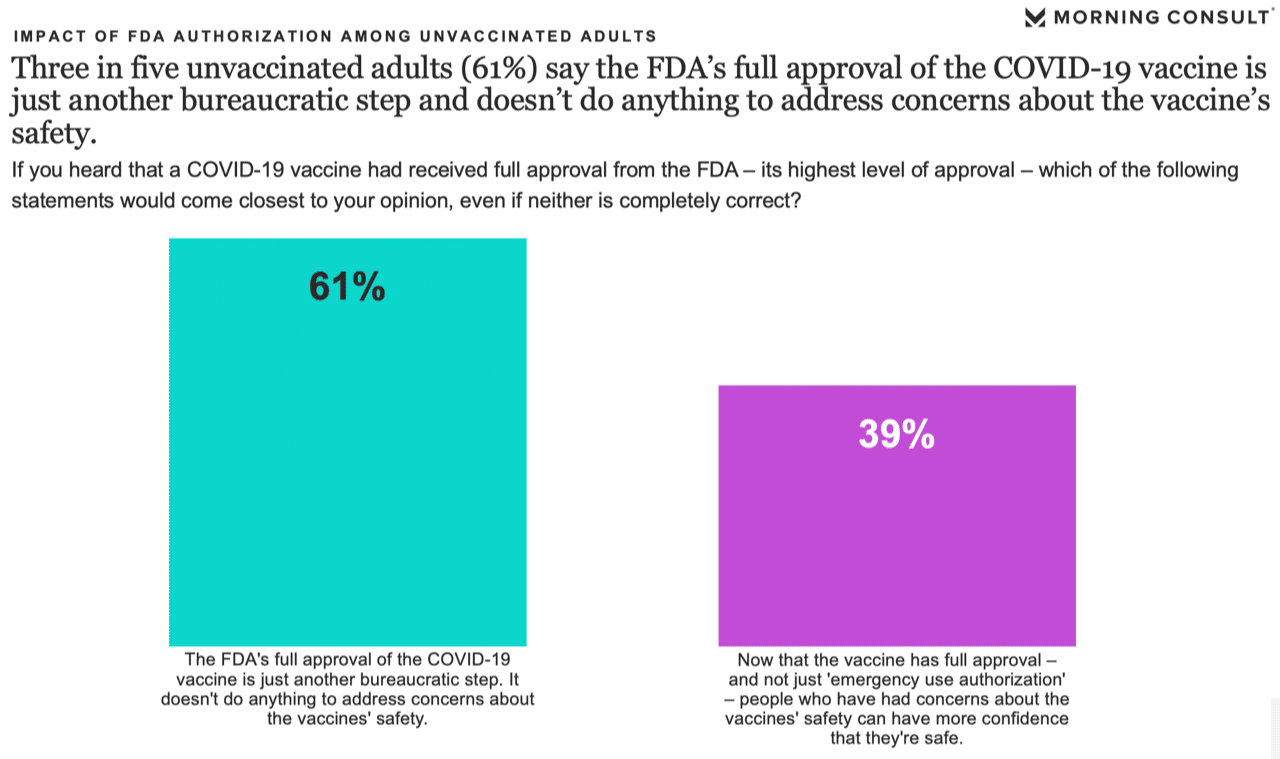
However, not all the findings were encouraging. When asked to choose between two statements, 61% of unvaccinated adults said the FDA’s approval is simply a bureaucratic step, compared with 39% who said approval should address people’s concerns about safety.
In a poll conducted the first week of August, more than half of unvaccinated parents said the FDA’s full approval would make them more confident in the vaccines and would make them more likely to get their children vaccinated. That poll also found that 81% of unvaccinated parents said they think vaccines for diseases like measles and mumps are safe for children, compared with only 60% who said the COVID-19 vaccines are safe for children.
Dr. Castrucci said, “Our message to parents should be: If you trust the safety of vaccines for measles and mumps, we have good news. The Pfizer COVID-19 vaccine now has the same level of approval as those other childhood vaccines.”
Methodology
On behalf of the de Beaumont Foundation, data intelligence company Morning Consult conducted an online survey between August 19-22, 2021 among a sample of 2,500 U.S. adults, including an oversample of 300 unvaccinated adults. Across the national sample and oversample, n=956 of unvaccinated adults. Results from the full survey have a margin of error of +/- 2%, and results among unvaccinated adults have a margin of error of +/- 3%.